UV Light in all its forms
All light is electromagnetic radiation and is commonly defined by its wavelength in nanometres (“nm”) and photon energy measured in electron volts (“eV”) or joules (“aJ”). We don’t normally think of visible light as radiation because we take for granted it is safe and a natural part of our environment. Yet, even visible light can be dangerous if it is too intense or inappropriately stimulates our hormonal system. For example, visible and near-visible blue light can disrupt our circadian rhythms.
UV light, by nature, has a higher energy level than visual wavelengths which is why it can destroy viruses, bacteria, and fungi. UV is also used to activate chemical processes and alter chemical structures. It stands to reason that materials, surfaces, and people can be negatively impacted by excessive UV exposure. This does not automatically mean that all UV is dangerous. Short-term exposure to “black lights” has been commonplace in entertainment venues like dance clubs, bars, and theatres as well as museums to create a “fluorescing” effect.
Short exposures to most UV is relatively safe, even to the naked eye and bare skin. There are several ranges within the UV category spanning from 10 nanometres (“nm”) to 457nm. Sanitizing UV usually holds between 457nm down to 180nm, spanning the most common categories as follows:
HOW UV-A WORKS
UV-A is the longest wavelength defined as ultraviolet spanning from 457nm to 315nm. While it has a lower energy than shorter wavelengths, it has greater penetrating power for skin and eyes. It is associated with sunburn, skin aging, and altering DNA in the dermis, epidermis, and even the hypodermis. Higher frequencies of UV-A from 425nm to 457nm usually require special lasers to be generated.
UV-A travels well through the atmosphere and is associated with modest germicidal potency. Some UV-A wavelengths can be generated by light emitting diodes (LEDs) and there are several sanitizing systems based upon this wavelength. All such systems require very long exposure periods that can be harmful to certain materials; in particular, polymers like plastics.
HOW UV-B WORKS
UV-B falls between 280nm and 315nm. It is associated with sunburn and skin cancers. It penetrates the dermis and can reach the epidermis.
Although UB-B has a higher energy than UV-A, some doctors and scientists believe it is more harmful for longer exposures.
Modest exposure of about 15 to 20 minutes of summertime UV-B has the benefit of generating vitamin D3 through a chemical reaction in the skin.
Most UV-B is filtered out at sea level by the atmosphere. Sun blocking lotions use waxes or metalized compounds like zinc oxide paste to prevent UV-B from penetrating the skin
HOW UV-C WORKS
UV-C is blocked from reaching the lower atmosphere by the ozone layer. Ranging from 200nm to 260nm, some UV-C at above 260nm to 280nm can reach very high altitudes, but is not naturally occurring from sunlight at sea level.
Within UV-C there are several categories, but the most common distinctions are between the germicidal frequency of 254nm and “far UV-C” falling between 207nm and 222nm. UV-C at 254/257nm is the most commonly used germicidal frequency (wavelength) due to its higher energy and proven efficacy. In general, light emitting diodes (LEDs) cannot generate germicidal wavelengths with sufficient intensity to sanitize wide areas. There are some LEDs being used to “clean air” with a circulation system, but surfaces must still be addressed using other methods.
HOW FAR UV-C WORKS
FAR UV-C references the 207nm to 222nm range and is highly effective in destroying viruses, bacteria, and fungi. Its higher energy is associated with germicidal power while the shorter wavelength is believed to be safe for human exposure because it does not penetrate the skin or eye cornea (lens).
It is important to note that assumed safety associated with the lack of penetration is based upon laboratory experiments. Since this frequency band is not naturally occurring in our environment, there is no way to determine with certainty that long-term exposure is not harmful. Still, claims are being made that continuous exposure to 207nm~222nm can be tolerated.
UV-C is absorbed by the atmosphere and does not travel well or far at sea level under normal humidity. This implies the far UV-C source must be relatively close to its intended target.
HOW VACUUM UV WORKS
VACUUM UV is often placed in the UV-C category, but it is significantly different because it is powerful enough to ionize substances/elements like oxygen (O2), turning it into ozone (O3). Vacuum UV ranges from 10nm to 200nm, but is usually confined between 180nm and 200nm which can be produced from special UV bulbs. It has very potent germicidal efficacy, but does not travel well through the atmosphere because it is absorbed and blocked when reacting to the atmosphere, creating ozone and other reactions. Because of the ozone effect, vacuum UV-can be effective in controlling dust mites, bed bugs, and certain insect larvae. Ozone is, itself, an effective germicide that destroys viruses, bacteria, fungi, and living organisms. Ozone fumigation is used in extermination and deodorization. In fact, ozone is perhaps the only effective means for removing odours as potent as skunk.
Professor Anne Rammelsberg of Millikan University explains that UV energy initiates a reaction between two thymine molecules within DNA. Although bacteria can normally repair damaged DNA, when the damage is extensive the cell ceases to function. This same response can be engendered in viruses and fungi relative to the wavelength used and the light intensity. There are actually four criteria that determine the germicidal “kill rate:”
1) UV spectrum/wavelength (UV-A, UV-B, UV-C, Vacuum UV)
2) Light intensity – power
3) Proximity to intended surface and/or space volume
4) Duration (exposure time)
In practice, effective sanitizing is a function of these four components taken proportionately. This means that a longer exposure can offset distance from the intended target and wavelength can determine exposure time as can intensity. The objective is to maximize efficiency and effectiveness in the shortest, least disruptive application. Since UV radiation can adversely affect plastics and materials, care must be taken to minimize possible damage. Here, the science is clear… short exposure is better because reactions occur over time.
UV DISTRIBUTION
Artificially generated visible light is usually directional, involving a symmetrical light source and a reflector. Consider a flashlight that sends a beam of light to a target. The reflector geometry directs the light beam. Light fixtures are designed to spread light at a desired intensity within the “field of view.” Sanitizing spaces like rooms or vehicles usually requires a different approach because the objective is to spread radiation in all directions rather than pointed or focused energy. This is because you couldn’t sanitize behind the light source. However, if the objective is to direct energy in a specific direction without other exposures, a focused approach makes sense. Examples include directing UV toward a food preparation table or a conveyer. Distributing UV from a central ceiling location can be effective if the energy distribution pattern is appropriate for the space and the particular surfaces within the space.
INVERSE SQUARE LAW
All light, including UV, loses intensity with distance. This is in accordance with the “inverse square law.” This is a critical consideration for UV because weaker light requires more exposure time. At some point, the sanitizing process is not feasible. Since each wavelength has a different energy level and propagates differently through air, the UV source must generate the most effective radiation using the least amount of energy and distribute that energy in accordance with the defined application. A stadium poses a different challenge than a hospital room or a vehicle. Spaces with high ceilings like convention centres and exhibition halls are not the same as hotel rooms.
UV IS DIRECTIONAL
Unlike visible light, UV is more directional. Consider that sunlight diffuses through a reflective process. That is why there is daylight in shaded areas. Natural light is distributed through reflection from surface to surface. The pattern of light distribution depends upon reflective surfaces. As previously mentioned, UV radiation is absorbed by materials. Generally, it does not reflect well. Moreover, when UV bounces off a surface, it can change wavelengths as well as lose energy. This is why reflectors are not effective for most UV applications
When scientific studies are aggregated, it appears that each UV wavelength contributes to the overall effectiveness of any sanitizing process. For example, UV-B wavelengths and higher are the only radiation that penetrate the Earth’s atmosphere to reach sea level. Yet, most UV sanitizers have, until recently, concentrated on UV-C at 254nm as the primary wavelength. Right behind this frequency is far UV-C from 207nm to 222nm, which some studies demonstrate better potency against some pathogens. The drawback is that the very nature that allegedly makes far UV-C safe when people are present, i.e. it does not travel as well as UV-B through the atmosphere, renders it ineffective in reaching modest distances from the source. Higher power levels can compensate for this deficiency; however, it is very difficult to produce high-power far UV-C radiation within the narrow bandwidth from 207nm to 222nm. Thus, most lamps claiming to produce this range have other radiation “leakage.” This adds an element of exposure risk. We know this because UV in that range is totally invisible, yet lamps that claim only this range still appear blue to the naked eye.
The two most common UV bulbs are fluorescent tubes and mercury vapor. Due to mercury’s toxic nature and environmental hazard, vapor bulbs were banned in the U.S. in 2008, leaving fluorescent. Although fluorescent and even magnetic induction bulbs contain mercury, levels are significantly lower than with mercury vapor lamps. Sterile-Bright™ bulbs use magnetic induction technology originally invented by Nikola Tesla in 1891.
Ozone is Different
Ozone is an unstable form of oxygen that is created when ionizing energy combines a third molecule to oxygen’s natural two-molecule state; O2 becomes O3. By nature, oxygen is a reactive element that “oxidizes” other elements. Ozone is often called “active oxygen” because it more rapidly oxidizes as it returns to its more stable O2 state. This is why ozone is such a good germicide, fungicide and deodorizer.
GENERATING OZONE
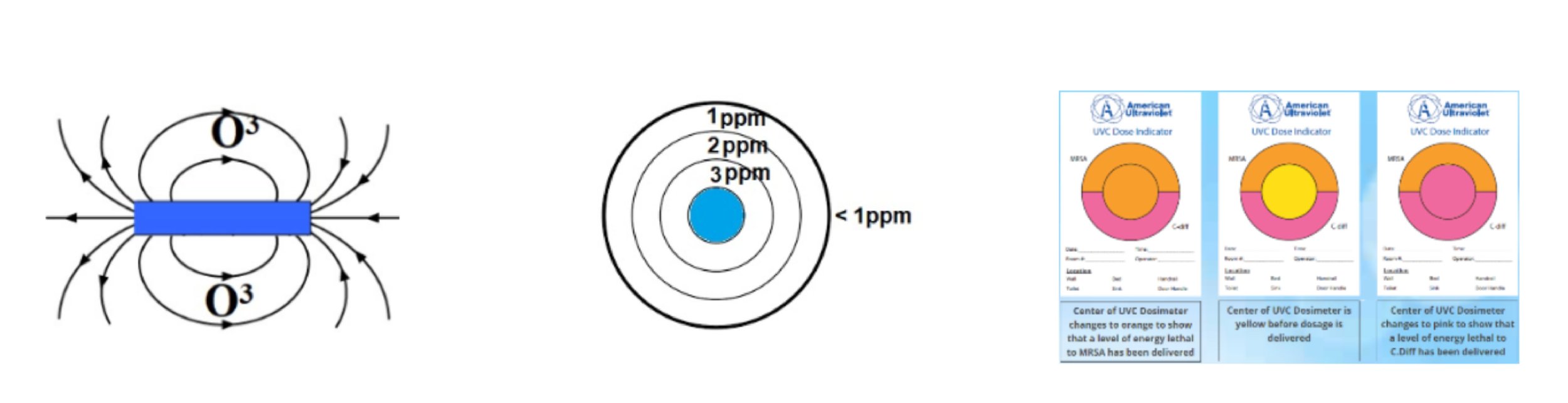
Sterile Bright™ units generate ozone as a consequence of ionizing vacuum UV. Unlike “ozonators” that make ozone using an electrostatic charge, Sterile Bright™ tubes create ozone from ionizing UV energy that radiates several feet away from the unit. Since Sterile-Bright™ fixtures are designed to provide radial 360-degree omnidirectional UV dispersion, ozone distribution does not require a fan. Still, fans or ventilation systems can be used to spread this germicidal gas.
Ozone has been used for sterilization for many decades because it is extremely effective at small concentrations. As little as 0.5 parts per million (ppm) of ozone can deactivate bacteria and viruses. Like most chemical treatments, the higher the concentration, the faster the process. Academic and technical papers suggest germicidal effects can require up to 25ppm for longer periods, however, ozone has the advantage of being an absolute gas, meaning it does not exist as a solid or liquid at room temperature. Thus, when ozone dissipates, its effect is completely gone. This is why ozone has been used instead of liquid and evaporative chemical treatments for sanitizing, deodorizing, and exterminating. For example, many hospitals and medical facilities have used, and continue using formaldehyde vaporization, peracetic acid, and/or chlorhexidine for sanitizing. These chemicals are caustic and toxic; they can cause many adverse reactions in humans and animals as well as damage surfaces and materials.
Ozone has the added advantage of being an irritant to insects and even deadly to bed bugs, dust mites, fleas, and lice. Insects and rodents instinctively flee from small ozone concentrations as little as 1ppm to 3ppm. This means that regular low-level ozone treatments can control pests which, themselves, can be disease carriers. In outdoor applications, ozone bonds to human odour molecules that would attract gnats and mosquitos.
Ozone air purification is debatable science. The Environmental Protection Agency (EPA) has labelled outdoor ozone as a pollutant, while acknowledging that “ozone high” is environmentally necessary, meaning our ozone layer protects us from harmful UV radiation. Ozone is routinely used to sanitize medical devices like ventilators, C-Pap and Bi-Pap sleep devices, and hospital ventilation systems. Ozone is also used to clear ductwork of mould and mildew as well as bacteria. A hot summer day can generate outdoor ozone levels that exceed EPA standards, making the entire effort to regulate ozone questionable. Although the EPA associates ground-level ozone with man-made pollution, natural events like sunlight and lightening create this gas which has been linked to general outdoor microbial reduction.
OZONE EXPOSURE TIME
Ozone is an irritant that can cause burning eyes and air passages. Excessive exposure can be harmful and cause reactions for people with compromised breathing like COPD and asthma. This is why Sterile-Bright™ exposure times are brief and the amount of ozone generated throughout a space does not exceed 1ppm during a customary treatment. Ozone creation declines as distance increases from the Sterile-Bright™ unit. At increments of three feet, ozone generation declines proportionally, depending upon the Sterile-Bright™ power which can range from 250 watts to 2,000 watts. Proper ozone protocols are achieved by following the appropriate exposure times required for each space. It is inevitably up to the operator to determine what levels of ozone are needed for the particular task. For example, if the objective is to discourage insect and rodent infestation in a food preparation or storage environment, higher levels of ozone may be desirable; i.e. kitchens, pantries, and refrigeration lockers.
Ozone is an extraordinarily effective deodorizer. As little as 0.01ppm can freshen air. The Occupational Safety and Health Administration (OSHA) guidelines call for no more than 0.1ppm exposure for 8 hours on a “time-weighted” average. This means that at 0.2ppm the acceptable exposure time is 4 hours and at 0.3ppm, 2 hours. Keep in mind that these are workplace exposure limits, meaning that an individual would be physically working in spaces where atmospheric ozone concentrations were at such levels. Ozone in excess of 5.0ppm is considered dangerous for any form of physical exertion.
WAVE LENGTH FORMULA
All light, including UV, loses intensity with distance. This is in accordance with the “inverse square law.” This is a critical consideration for UV because weaker light requires more exposure time.
At some point, the sanitizing process is not feasible. Since each wavelength has a different energy level and propagates differently through air, the UV source must generate the most effective radiation using the least amount of energy and distribute that energy in accordance with the defined application.
A stadium poses a different challenge than a hospital room or a vehicle. Spaces with high ceilings like convention centres and exhibition halls are not the same as hotel rooms.